Make your Life Easier with the PMCF documents, ready for use in the Resource Center
Introduction
Post-Market Clinical Follow-Up (PMCF) is a critical requirement under the Medical Device Regulation (MDR) 2017/745, ensuring that medical devices remain safe and effective throughout their lifecycle. For medical device manufacturers—especially startups and small to medium-sized companies—understanding and implementing a robust PMCF strategy is essential for regulatory compliance, risk mitigation, and market success.
What is PMCF?
Post-Market Clinical Follow-Up (PMCF) is a proactive, systematic process of gathering and evaluating real-world clinical data to confirm the safety, performance, and benefit-risk profile of a medical device once it is on the market.
PMCF is a fundamental component of Post-Market Surveillance (PMS) and is required for all Class I, IIa, IIb, and III medical devices under MDR. Even for lower-risk devices, PMCF is still be necessary for the implementation of general PMCF procedures and methods.
Why is PMCF Important?
This guide covers key PMCF elements, regulatory expectations, and best practices, helping manufacturers develop a compliant and efficient PMCF process.
- Ensures ongoing compliance with MDR
- Identifies unknown risks or long-term effects not captured during pre-market studies
- Supports clinical evaluation updates by providing real-world evidence (RWE)
- Enhances patient safety and device performance through continuous monitoring
- Prevents regulatory issues, recalls, or penalties by demonstrating proactive compliance
Key Elements of a PMCF Plan
To ensure compliance with MDR and MDCG guidance (MDCG 2020-7 and MDCG 2020-8), manufacturers must implement a well-defined PMCF Plan that includes:
1. General & Specific PMCF Methods
A comprehensive PMCF strategy involves both general and specific methods:
✅ General PMCF Methods:
– Literature reviews (systematic screening of clinical studies in databases like PubMed, Embase, Cochrane)
– Analysis of vigilance reports and adverse event databases (FDA MAUDE, EUDAMED, MHRA, ANSM)
– Feedback collection from users (physicians, patients, distributors, social media)
– Patient or User Surveys: Collecting feedback on usability, performance, and safety without specific concerns
✅ Specific PMCF Methods:
– PMCF Investigations: Prospective clinical studies designed to gather new clinical evidence
– Patient or User Surveys: Collecting feedback on usability, performance, and safety for specific concerns
– Device Registries: Tracking real-world device performance in specific patient populations
2. PMCF Objectives
PMCF should aim to:
– Confirm device safety and effectiveness in real-world conditions until the end of the lifetime
– Identify emergent risks, previously unknown risks and side effects
– Ensure the benefit-risk profile remains acceptable
– Detect potential off-label use or systematic misuse
3. Link to Risk Management & Clinical Evaluation
PMCF findings must directly inform and update:
– Clinical Evaluation Reports (CER)
– Risk Management Files
– Periodic Safety Update Reports (PSUR)
– Summary of Safety and Clinical Performance (SSCP) for Class III & Implantables
4. Timelines & Reporting
Manufacturers should align PMCF activities with PMS reporting schedules:
– Class III & IIb Devices → Annual PMCF Reports
– Class IIa Devices → Every 2 years
– Class I Devices → Every 3 to 5 years, with a justification
PMCF Evaluation Report: What Should It Include?
The PMCF Evaluation Report (PMCF ER) consolidates findings from the PMCF Plan, documenting the impact on clinical evaluation and risk management.
📌 Key Sections of a PMCF Evaluation Report:
– Device & Manufacturer Details
– Summary of PMCF Activities Conducted
– Data Analysis & Interpretation
– New or Emerging Risks Identified
– Impact on Risk Management & Clinical Evaluation
– Conclusions & Next Steps
Note: refer to MDCG guidance (MDCG 2020-7 and MDCG 2020-8) for further information
Best Practices for an Effective PMCF Strategy
To ensure compliance and efficiency, manufacturers should:
✔ Integrate PMCF with PMS & Risk Management
✔ Use a structured approach (MDCG 2020-7 & 2020-8 templates)
✔ Leverage real-world data (RWD) & Real-World Evidence (RWE)
✔ Collaborate with clinicians & regulatory experts
✔ Maintain documentation for audits & notified body reviews
Common PMCF Challenges & How to Overcome Them
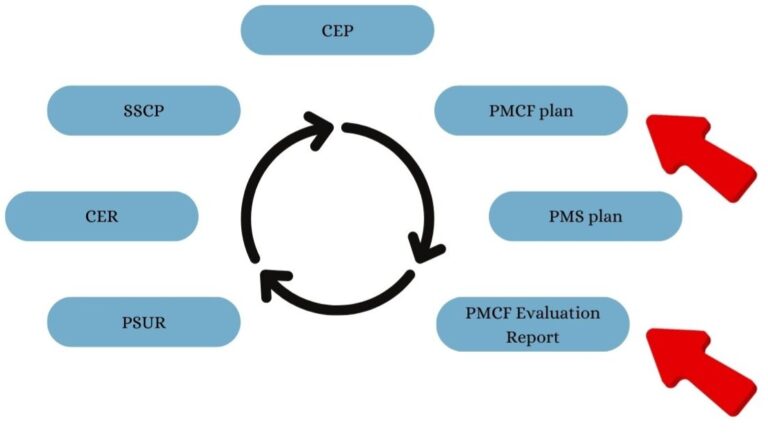
PMS Lifecycle
The Post-Market Surveillance (PMS) lifecycle is a continuous process ensuring that medical devices remain safe, effective, and compliant throughout their market presence. As shown in the cycle, PMCF plays a critical role by proactively gathering real-world clinical data to update the Clinical Evaluation Report (CER), Periodic Safety Update Report (PSUR), and Summary of Safety and Clinical Performance (SSCP).
⚠ Be careful! A common mistake is to think about the PMCF schedule in isolation without considering PMS and Clinical Evaluation activities globally. This misalignment often leads to delays and an inability to meet announced timelines, making it impossible to comply with MDR-defined deadlines. To ensure a cohesive and compliant strategy, PMCF must be planned in synchronization with the entire PMS lifecycle.
Conclusion: Stay Compliant, Stay Competitive
A strong PMCF strategy is not just a regulatory requirement—it is an opportunity to enhance patient safety, product performance, and market credibility.
By systematically collecting and analyzing clinical data, medical device manufacturers can avoid compliance risks, improve device safety, and gain a competitive edge in the evolving regulatory landscape.
👉 Need ready-to-use PMCF templates?
Download the PMCF Plan, PMCF Procedure & PMCF Evaluation Report Templates from LEXQARA’s Resource Center.
🔹 Make Your Life Easier with LEXQARA’s Regulatory Solutions!
📞 Contact us: +33 (0)6 87 45 88 07
📧 Email: alexandre.petiard@lexqara.com
Alexandre Pétiard, President, LEXQARA
